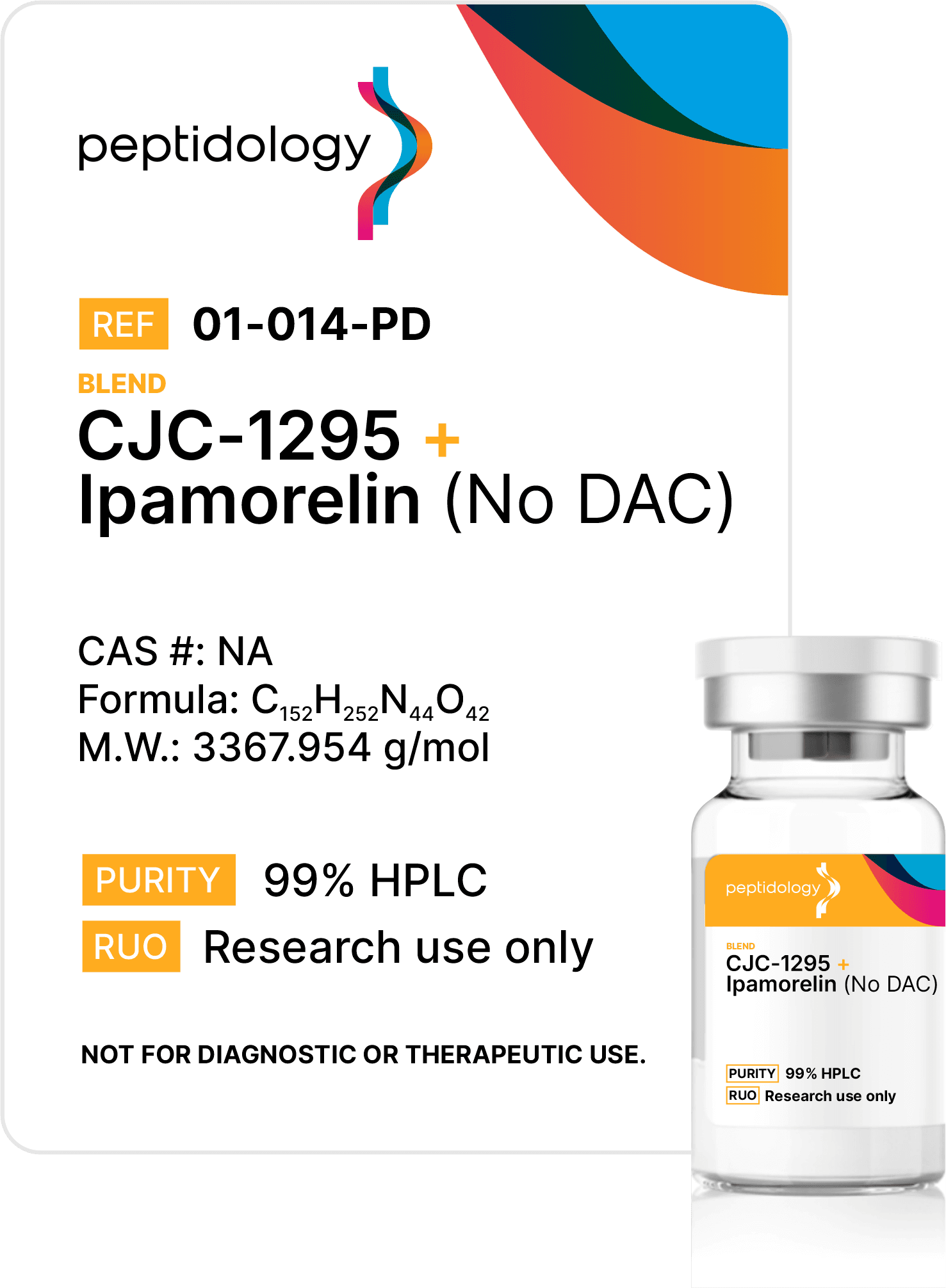
CJC 1295 no DAC / Ipamorelin
$64.00
Compound background
CJC-1295 (no DAC) / Ipamorelin Blend pairs a short-acting 29-residue GHRH analog with a highly selective pentapeptide GHS-R1a agonist to amplify physiologic, pulse-like growth-hormone release. This product contains CJC-1295 No DAC (5mg) and Ipamorelin (5mg) blended together in a single vial.
Quality standards
- Greater than 99% pure ✅
- LPS/Endotoxins ✅
- Heavy metals ✅
- Sterility guaranteed ✅
Sourcing standards
- Raw ingredients manufactured by an FDA registered & audited supplier following cGMP standards
- Finished peptides 0.22 µm sterile-filtered (aseptically processed) and produced in a GMP facility
- Tested by a US FDA registered & audited third-party analytical lab accredited by ISO/IEC and U.S. Department of Ecology
Learn more about Peptidology product sourcing, testing and quality standards.
Analytical reports
Structure & Specification
CJC-1295 no DAC 5mg / Ipamorelin 5mg Blend: Premier Research Peptide Combination
Disclaimer: This product is intended solely for laboratory research purposes. It is not suitable for consumption by humans, nor for medical, veterinary, or household purposes. Researchers should handle CJC1295/Ipamorelin with care and adhere to strict safety guidelines during experiments. Kindly review our Terms & Conditions before making a purchase. *
Money Back Guarantee
Satisfaction Guarantee
Easy Returns
Secure Ordering
Us vs The Competition

- Competitive Pricing
- Third-Party Tested Verified COAs
- Same Day Shipping Fast Delivery
- Overpriced
- No Testing or First Party Only Testing
- Same Day Shipping Fast Delivery
Always quality-tested, verified with third party COA’s
At every step, we prioritize quality by conducting rigorous third-party testing on all our products. These tests focus on four key characteristics—identity, purity, sterility, and endotoxicity—ensuring that each batch meets the highest standards for safety and performance, with independent third-party Certificates of Analysis (COAs) to verify our commitment to excellence.
Identity Test
Identity testing ensures that the product contains the correct ingredient as labeled, verifying its authenticity and matching it to established reference standards.
Purity Test
Purity and concentration testing verifies that the ingredient is present in the correct amount, with a purity of 98% or higher to meet stringent quality standards.
Sterility Test
Sterility testing ensures that the product is completely free from bacteria, fungi, and other microorganisms.
Endotoxicity
Endotoxicity testing specifically detects and quantifies lipopolysaccharides (LPS), components of bacterial cell walls, to ensure the product is free from harmful endotoxins.