AOD-9604
Original price was: $69.99.$59.99Current price is: $59.99.
Analytical lab reports
Batch 1666 – AOD-9604 99.25% 10.38mg
Note: A cloudy reconstitution of AOD-9604 is expected behavior as it has a lower intrinsic water solubility and higher tendency to form colloidal suspensions rather than true solutions. AOD-9604 may prefer a slightly acidic or buffered solution to dissolve fully. Bacteriostatic water (pH ~5.7) or saline might not always hit the optimal pH for full dissolution, causing partial clumping. The product is sterile as confirmed by its COA.
Research Use Only (RUO) — In-Vitro Laboratory Use Only
This research-grade analytical standard is produced for controlled laboratory environments and supplied exclusively for in-vitro research. Each lot is manufactured with batch-level traceability and released only after structured, multi-point quality verification. Not for human or veterinary use.
- Intended solely for laboratory research (RUO / in-vitro only)
- Lot-specific Certificate of Analysis (CoA) with batch traceability
- ≥99% purity reported per lot (see CoA)
- 7-point testing program (analytical identity/purity plus additional quality verification per lot)
- Endotoxin and elemental impurity screening reported per lot (see CoA)
- Independent third-party verification, including two-lab microbial contamination testing (documentation available)
- Sealed, tamper-evident packaging to help maintain compound integrity during storage and handling
Testing metrics and documentation are provided for analytical characterization and research reproducibility only and do not imply safety or suitability for any other use.
Structure & Specification
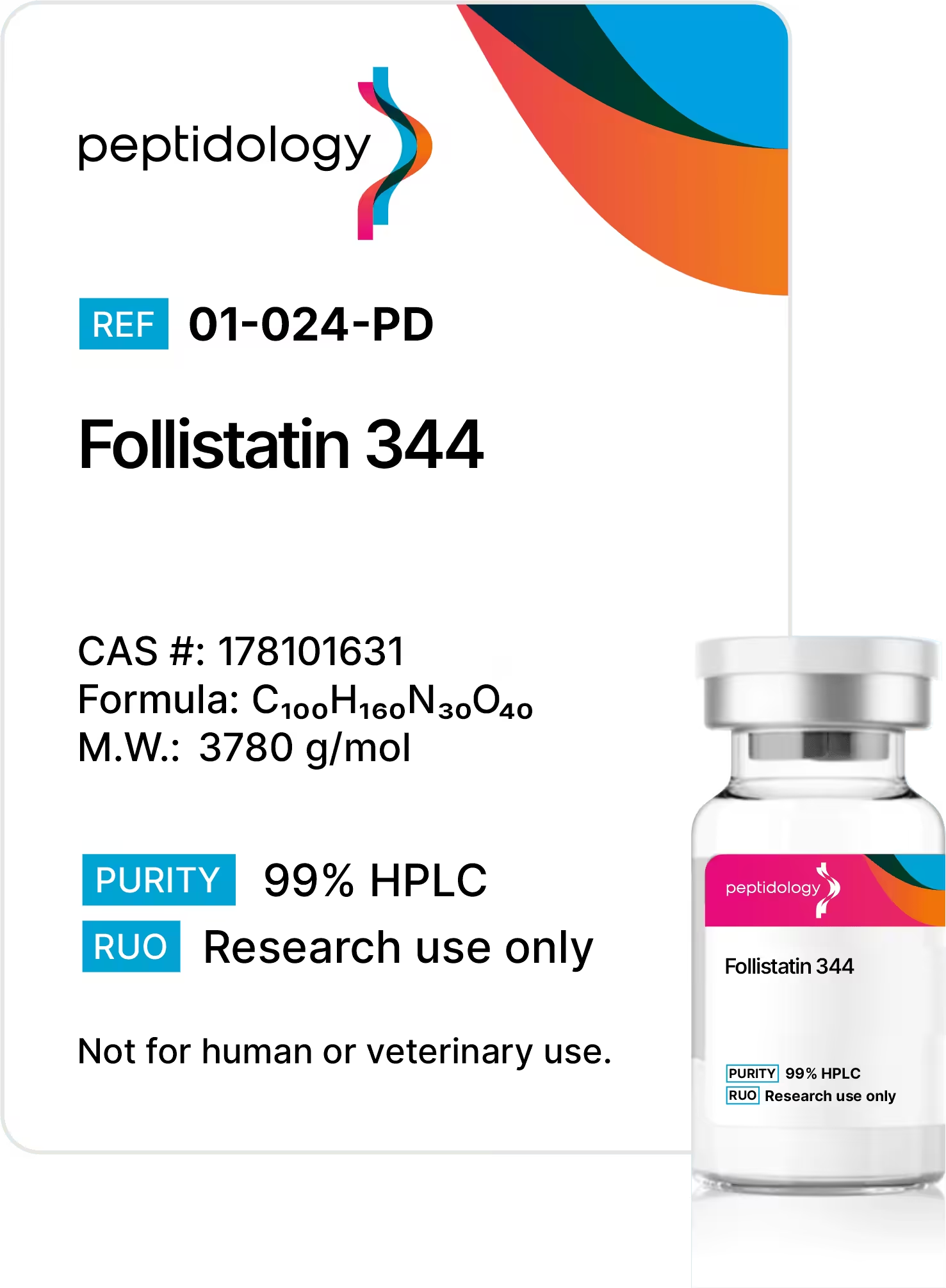
AOD-9604 Peptide Summary
Product Specifications: Lyophilized Powder (>99%+ purity) in 3ml vial.
*Requires Reconstitution with solvent such as BAC Water (Sold Here)
Appearance: Solid, white powder in 3mL glass ampule
Chemical Formula: C78H123N23O23S2
PubChem CID: 71300630
CAS Number: 386264-39-7
Molecular Weight: 1815.12 g/mol
Storage: Store at ≤6°C, sealed, away from heat, light and moisture.
Disclaimer: This product is intended solely for laboratory research purposes. It is not suitable for consumption by humans, nor for medical, veterinary, or household purposes. Researchers should handle AOD-9604 with care and adhere to strict safety guidelines during experiments. Kindly review our Terms & Conditions before making a purchase. *
Money Back Guarantee
Satisfaction Guarantee
Easy Returns
Secure Ordering
Us vs The Competition

- Competitive Pricing
- Third-Party Tested Verified COAs
- Same Day Shipping Fast Delivery

- Overpriced
- No Testing or First Party Only Testing
- Same Day Shipping Fast Delivery
Always quality-tested, verified with third party COA’s
At every step, we prioritize quality by conducting rigorous third-party testing on all our products. These tests focus on four key characteristics—identity, purity, sterility, and endotoxicity—ensuring that each batch meets the highest standards for safety and performance, with independent third-party Certificates of Analysis (COAs) to verify our commitment to excellence.
Identity Test
Identity testing ensures that the product contains the correct ingredient as labeled, verifying its authenticity and matching it to established reference standards.
Purity Test
Purity and concentration testing verifies that the ingredient is present in the correct amount, with a purity of 98% or higher to meet stringent quality standards.
Sterility Test
Sterility testing ensures that the product is completely free from bacteria, fungi, and other microorganisms.
Endotoxicity
Endotoxicity testing specifically detects and quantifies lipopolysaccharides (LPS), components of bacterial cell walls, to ensure the product is free from harmful endotoxins.