Klotho Research Peptide and KP1: The Klotho Fragment
Research-only legal/ethical disclaimer (read first): This article is intended solely for licensed researchers and is provided for educational purposes only. The peptides and protein constructs discussed (including Klotho research peptide and the Klotho fragment KP1) are not approved for human or animal use in this context, and nothing here is a recommendation to perform in vivo (human or animal) testing. Any discussion of biological effects, contraindications, or adverse outcomes refers only to published, peer‑reviewed research (including in vitro and previously published in vivo studies).
Figure 1. Structural snapshot of human α‑Klotho (cryo‑EM entry 8UF8), illustrating the folded ectodomain architecture underlying receptor interactions.
(RCSB PDB)
1) Why Klotho became an “aging” headline—without being magic
Klotho (α‑Klotho; gene KL) entered modern biogerontology through an arresting observation: disrupting Klotho expression in mice produced a cluster of phenotypes that resemble accelerated aging. The original Nature report described that “a defect in klotho gene expression in the mouse results in a syndrome that resembles human ageing,” including short lifespan and multi‑system pathology. (Nature)
That discovery seeded two parallel research tracks that continue today:
- Physiology-first Klotho: Klotho as a kidney- and brain-enriched regulator of mineral metabolism and endocrine signaling (notably with FGF23). (PMC)
- Intervention-first Klotho: efforts to reproduce selected Klotho functions using recombinant domains, fragments, or short peptides—motivated by the practical constraints of the full-length protein. (PMC)
A useful framing from a major review is that Klotho may act as an “integrator” across organ systems; the authors note that genetic overexpression can extend lifespan in mice, while Klotho deficiency associates with multiple age-related disease states. (PMC)
2) What “Klotho research peptide” means in molecular terms
Although commonly discussed in “longevity” language, α‑Klotho is fundamentally a single-pass transmembrane protein with a large extracellular ectodomain containing two homologous regions (KL1 and KL2). It exists in membrane-bound form and in circulating/soluble forms generated by shedding or alternative processing (terminology varies across papers and assays). (RCSB PDB)
In research settings, the phrase Klotho research peptide is often used loosely to refer to recombinant Klotho domains or Klotho-derived peptides (short amino-acid fragments) used to probe mechanisms. The key scientific motivation is captured directly in the KP1 discovery paper: Klotho is “a large transmembrane protein,” and that scale/complexity can make it hard to “harness…as a therapeutic remedy,” motivating peptide-based mimics. (PMC)
3) The canonical axis: FGF23–Klotho in phosphate homeostasis
The most established physiologic role of α‑Klotho is as an obligate co-receptor that enables FGF23 to signal effectively through specific FGF receptors in target tissues (especially kidney). In an NIH-hosted review, the mechanism is summarized crisply: “The availability of an adequate amount of Klotho is essential for FGF23 to exert its phosphaturic effects in the kidney.” (PMC)
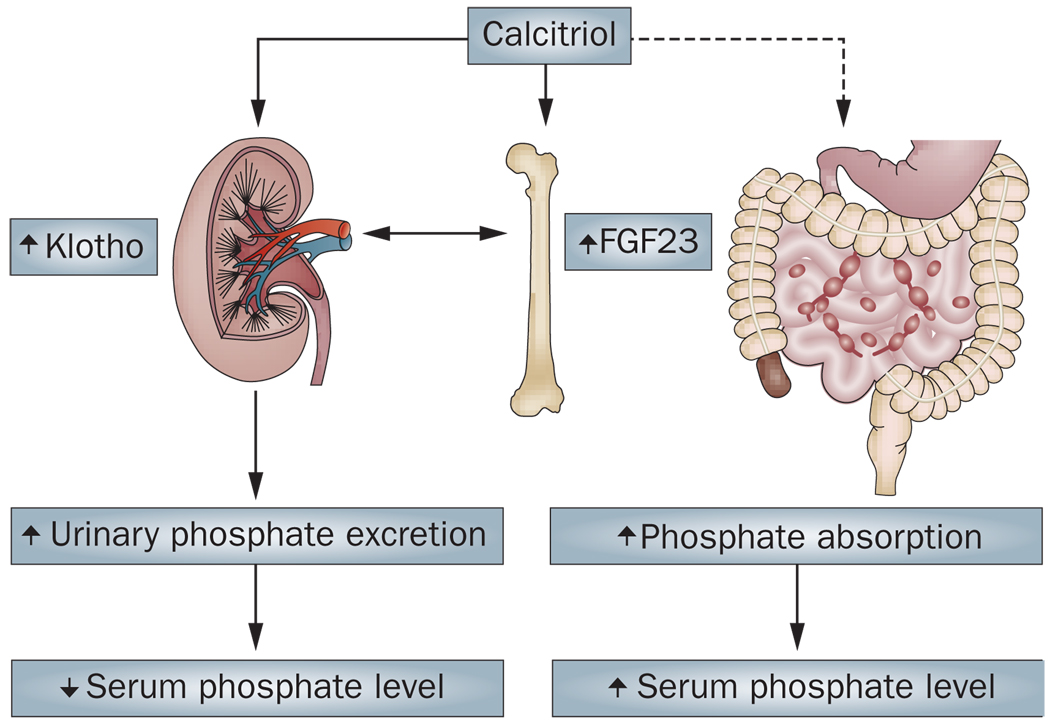
Figure 2. Conceptual map of the FGF23–Klotho–vitamin D network regulating phosphate handling.
(NCBI)
In this axis, FGF23 (bone-derived) and Klotho (kidney-enriched) coordinate signals that regulate phosphate transporters and vitamin D metabolism, thereby influencing serum phosphate levels and downstream vascular/skeletal consequences when dysregulated. (PMC)
This physiology matters for peptide/fragment work for two reasons:
- It provides biomarker and phenotype anchors (phosphate, vitamin D metabolites, vascular calcification risk) that can act as readouts in experimental systems. (PMC)
- It creates a risk envelope: altering Klotho/FGF23 signaling can, in principle, perturb mineral metabolism. The KP1 discovery paper itself highlights that very high Klotho levels have been associated with disturbed calcium/phosphate metabolism (e.g., hypocalcemia/hypophosphatemia) in prior work, underscoring why “partial mimics” are attractive. (PMC)
4) Beyond phosphate: pleiotropic signaling nodes linked to aging biology
A recurring theme across Klotho reviews is that soluble or domain-based Klotho activity touches several pathways repeatedly implicated in fibrotic remodeling, inflammation, oxidative stress responses, and cellular senescence. One open review emphasizes Klotho’s inhibitory influence on pathways including TGF‑β, Wnt/β‑catenin, and inflammatory signaling. (Frontiers)
Importantly, these “anti-aging” descriptions are best understood as network effects: Klotho’s most compelling mechanistic stories often show up in organ-specific injury contexts (kidney, lung, brain) where fibrosis, inflammation, and senescence intersect.
Kidney: the epicenter of α‑Klotho biology
α‑Klotho is highly expressed in renal tubular epithelium, and many models of kidney injury show reduced Klotho expression. In CKD, Klotho loss can be both a marker and a potential driver of progression in some experimental settings. (PMC)
Lung: an example of Klotho decline in human disease tissue
In idiopathic pulmonary fibrosis research, investigators assessed Klotho levels in human plasma and primary lung fibroblasts from affected patients and reported downregulation; transgenic overexpression in mice showed protection in that model system. (PubMed)
Human observational data: association, not proof
Large epidemiologic analyses can’t establish causality, but they help define where Klotho behaves like a meaningful biomarker. An open-access Scientific Reports (Nature Portfolio) study using NHANES data (2007–2016) reported associations between higher serum α‑Klotho and lower prevalence of several cardiometabolic conditions (with noted non-linear relationships for some outcomes). (Nature)
5) KP1 (Klotho-derived peptide 1): what it is and why it’s different
KP1 is a 30–amino acid peptide derived from the KL1 region of human Klotho. It was identified by screening overlapping KL1-derived fragments for the ability to block pro-fibrotic signaling in kidney cell systems. (PMC)
The central claim of the discovery paper is straightforward and directly stated in the abstract: “Here we report the discovery of a Klotho-derived peptide 1 (KP1) protecting kidneys by targeting TGF-β signaling.” (PMC)
Mechanism: KP1 targets the “front door” of TGF‑β signaling
The mechanistic novelty is that KP1 appears to bind TGF‑β receptor 2 (TβR2) and disrupt ligand–receptor engagement, thereby reducing downstream pro-fibrotic pathways (Smad-dependent and MAPK branches were examined in the original work). (PMC)
This matters because TGF‑β is a major node in fibrotic remodeling across organs, and broad TGF‑β blockade has historically raised safety and specificity concerns in translational medicine. KP1 is conceptually positioned as a Klotho-like modulator that constrains TGF‑β signaling while (hypothetically) avoiding some systemic liabilities of full-length Klotho elevation. (PMC)

Figure 3. KP1 identification includes high sequence conservation across species and experimental readouts consistent with reduced fibroblast activation in vitro.
(PMC)
6) KP1 and cellular senescence: a second mechanism emerges
Follow-on studies expanded KP1’s profile from “anti-fibrotic peptide mimic” to a candidate anti-senescence modulator in kidney injury contexts. In a peer-reviewed report, the authors state: “we demonstrate that KP1… inhibits cellular senescence by restoring endogenous Klotho expression.” (PMC)
Mechanistically, this work proposes a post-transcriptional regulatory circuit involving miR‑223‑3p and lncRNA‑TUG1, in which KP1 shifts non-coding RNA dynamics in a way that favors restoration of Klotho protein expression and correlates with lower senescence markers in injured kidney tissue. (PMC)
From a research design standpoint, this creates an important experimental question: is KP1 primarily (a) a direct TβR2 antagonist, (b) a Klotho expression “inducer,” or (c) both, depending on context, timing, and cell type? The authors themselves flag that additional mechanisms and RNA regulators could be involved. (PMC)
7) KP1 in viral-associated kidney injury models (published research context)
A separate peer-reviewed study explored Klotho deficiency and KP1 in the context of SARS‑CoV‑2–associated kidney injury models. The conclusion frames Klotho loss as a key susceptibility factor: “Klotho deficiency is a key determinant of developing COVID-19-associated AKI.” (PMC)
In vitro, the study reported that expression of SARS‑CoV‑2 nucleocapsid protein induced injury and senescence-associated markers in kidney cell systems, and that KP1 and Klotho were associated with mitigation of those lesions within the reported experimental setup. (PMC)
Two careful takeaways for researchers:
- This line of work ties KP1 to a stress/injury amplification loop (aging/CKD → low Klotho → vulnerability to additional insults). (PMC)
- It also illustrates a general limitation: when a peptide shows multi-pathway effects in complex models, disentangling direct receptor binding from indirect gene-regulatory consequences becomes the central validation challenge.
8) Klotho fragments beyond KP1: brain resilience as a case study
KP1 is not the only Klotho fragment explored experimentally. A prominent example is a larger α‑Klotho fragment used in neuroscience research that produced cognitive and resilience phenotypes in mouse studies (again: published in vivo evidence, not a recommendation for use). The paper’s framing captures the surprising aspect of the result: it “surprisingly induced cognitive enhancement and neural resilience despite impermeability to the blood-brain barrier.” (PMC)
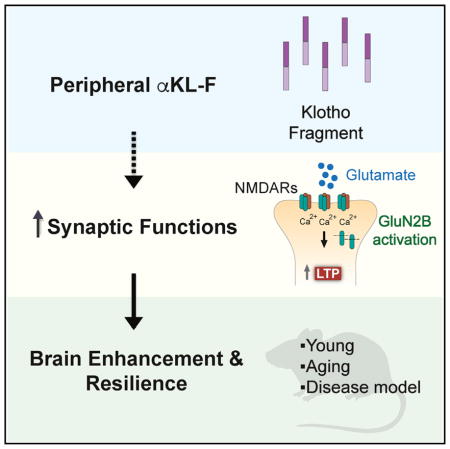
Figure 4. A graphical overview from published research linking an α‑Klotho fragment to synaptic function and resilience mechanisms.
(NCBI)
This broader fragment work is relevant to KP1 because it reinforces a general principle: Klotho biology can be “modular.” Different segments of the protein may preserve (or reshape) subsets of Klotho’s broader signaling repertoire, depending on what binding interfaces are retained. (PMC)
9) Contraindications, side effects, and experimental safety signals (research framing)
Because your instructions explicitly allow discussion of contraindications/side effects only when grounded in peer-reviewed research, here are the most defensible “risk hypotheses” researchers track in the Klotho/KP1 space:
- Mineral metabolism disruption (Klotho axis): Full-length Klotho modulation intersects phosphate and calcium regulation; the KP1 discovery paper explicitly notes concerns about mineral disturbances with high Klotho levels, motivating smaller mimics. (PMC)
- TGF‑β pathway interference (KP1 axis): Because TGF‑β signaling participates in wound repair, immune regulation, and cancer biology, any agent that disrupts TGF‑β receptor engagement raises context-dependent safety questions—even if it is more targeted than global inhibition. (KP1’s proposed mechanism is receptor-level interference.) (PMC)
- Assay and antibody validity: Klotho measurement is notoriously sensitive to assay choice, isoform handling, and antibody specificity—an issue emphasized in methodological discussions across the field and highlighted as a translational barrier in reviews. (PMC)
For investigators designing in vitro studies, these risks translate into practical controls: mineral signaling readouts when studying “Klotho-like” interventions, pathway specificity checks (Smad vs. MAPK branches), and orthogonal validation of Klotho protein changes (not only mRNA). (PMC)
10) Open questions that define the next wave of KP1/Klotho research peptide work
The KP1 and Klotho research peptide literature has matured to the point where the bottleneck is less “does it do anything in a model?” and more “what, exactly, is the causal chain?”
High-value open questions include:
- Binding specificity and structure: What is the precise KP1–TβR2 interaction surface, and does it vary by receptor conformation or cell context? (PMC)
- Modularity vs pleiotropy: Which Klotho functions can be cleanly reproduced by fragments (KL1/KL2-derived) without perturbing mineral metabolism? (PMC)
- Endogenous Klotho restoration: In senescence-focused models, is the most important effect KP1’s direct TGF‑β interference, its miRNA/lncRNA-mediated Klotho restoration, or both? (PMC)
- Human relevance: Observational associations (e.g., NHANES-based findings) support biomarker relevance, but intervention claims require far more evidence and careful endpoint selection. (Nature)
11) A brief note on “peptide therapy” language vs research reality
Commercial and clinical websites often use “peptide therapy” as an umbrella term for diverse compounds and goals. For example, GeneMedics describes peptides as naturally occurring signaling molecules and frames “peptide hormone therapy” as an attempt to “restore” peptide signaling with age. (genemedics.com)
From a scientific standpoint, the Klotho/KP1 story is narrower and more demanding: it hinges on defined sequences, defined targets (FGF23–Klotho endocrine axis; TβR2/TGF‑β signaling), and replicable mechanistic validation in controlled experimental systems. (PMC)
References (APA-style; URLs)
Barnes, J. W., et al. (2019). Role of fibroblast growth factor 23 and Klotho cross talk in idiopathic pulmonary fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology. https://journals.physiology.org/doi/full/10.1152/ajplung.00246.2018 (Physiology Journals)
Cheikhi, A., et al. (2019). Klotho: An elephant in aging research. Frontiers in Aging Neuroscience. https://pmc.ncbi.nlm.nih.gov/articles/PMC7330474/ (PMC)
GeneMedics. (2024). Peptide therapy. https://www.genemedics.com/peptide-therapy (genemedics.com)
Kuro-o, M., et al. (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature, 390(6655), 45–51. https://www.nature.com/articles/36285 (Nature)
Leon, J., et al. (2017). Peripheral elevation of a Klotho fragment enhances brain function and resilience in young, aging, and α-synuclein transgenic mice. Cell Reports. https://pmc.ncbi.nlm.nih.gov/articles/PMC5816951/ (PMC)
Martin, A., David, V., & Quarles, L. D. (2012). Regulation and function of the FGF23/Klotho endocrine pathways. Physiological Reviews. https://journals.physiology.org/doi/abs/10.1152/physrev.00002.2011 (Physiology Journals)
Razzaque, M. S. (2009). The FGF23–Klotho axis: endocrine regulation of phosphate homeostasis. Nature Reviews Endocrinology, 5(11), 611–619. https://pmc.ncbi.nlm.nih.gov/articles/PMC3107967/ (PMC)
Razzaque, M. S. (2009). FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? American Journal of Physiology-Renal Physiology. https://journals.physiology.org/doi/abs/10.1152/ajprenal.90538.2008 (Physiology Journals)
RCSB Protein Data Bank. (2024). 8UF8: Cryo-EM structure of alpha-Klotho. https://www.rcsb.org/structure/8UF8(RCSB PDB)
Wang, K., & Liu, J. (2025). Anti-aging protein α-Klotho is potential for reducing comorbidity risk of cardiometabolic diseases in vulnerable populations and enhancing long-term prognosis. Scientific Reports, 15, 16722. https://www.nature.com/articles/s41598-025-01580-4 (Nature)
Yuan, Q., et al. (2022). A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nature Communications, 13, 438. https://www.nature.com/articles/s41467-022-28096-z (Nature)
Yuan, Q., et al. (2022). A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nature Communications (PMC full text). https://pmc.ncbi.nlm.nih.gov/articles/PMC8782923/ (PMC)
Xu, J., et al. (2024). Klotho-derived peptide KP1 ameliorates SARS-CoV-2-associated acute kidney injury. Frontiers in Pharmacology (PMC full text). https://pmc.ncbi.nlm.nih.gov/articles/PMC10795167/ (PMC)
Zhang, X., et al. (2024). Klotho-derived peptide 1 inhibits cellular senescence in the fibrotic kidney by restoring Klotho expression via posttranscriptional regulation. Theranostics, 14, 420–?. https://pmc.ncbi.nlm.nih.gov/articles/PMC10750200/ (PMC)