Introduction
AOD-9604 is a synthetic peptide fragment derived from human growth hormone (HGH), specifically corresponding to the amino acid region 177–191 of HGH with an additional tyrosine added at the N-terminus for stability lexology.com . This modified fragment – often referred to as the “AOD-9604 research peptide” – was originally investigated for its fat-reducing properties. It was designed to retain the lipolytic (fat-burning) activity of full-length HGH without the hormone’s growth-promoting or other adverse effects. Over the past two decades, AOD-9604 has been the subject of extensive research, including preclinical studies in cell and animal models and clinical trials in humans. Researchers have examined its molecular mechanism of action, therapeutic potential in obesity and metabolic disorders, and even exploratory uses in tissue repair. This article provides a comprehensive scientific overview of AOD-9604 for licensed researchers, covering its development history, molecular background, current research findings, comparisons with natural HGH and other HGH fragments, as well as documented benefits and safety profile. All information is drawn from peer-reviewed studies, and no dosing or usage recommendations are provided (as AOD-9604 remains unapproved for clinical use).
History of AOD-9604 Development
AOD-9604 (short for “Anti-Obesity Drug 9604”) was first developed in the 1990s in Australia in the laboratory of Professor Frank Ng, a biochemist who had long studied growth hormone biology. By 1997, Ng and colleagues had identified the C-terminal segment of HGH (residues 177–191) as a key region for fat metabolism, and they created AOD-9604 by adding a tyrosine residue to the fragment to increase its stability lexology.com . A patent for this peptide was filed in 1997 and granted in 2003, claiming the specific 16-amino-acid sequence Tyr-Leu-Arg-Ile-Val-Gln-Cys-Arg-Ser-Val-Glu-Gly-Ser-Cys-Gly-Phe. The development was spearheaded by an Australian biotech company,
Metabolic Pharmaceuticals, which aimed to commercialize AOD-9604 as an anti-obesity drug. Between the late 1990s and mid-2000s, at least six human clinical trials were conducted, enrolling a total of over 900 participants. These included Phase 2 trials examining weight loss efficacy and safety. Early-phase results were promising in terms of safety and modest weight reduction, but larger trials failed to show statistically significant clinical weight loss across the broad population. By February 2007, the obesity development program was terminated due to lack of “clinically meaningful weight loss” despite AOD-9604’s excellent safety profile lexology.com . After 2007, AOD-9604’s developers pivoted to explore other potential applications. There were exploratory efforts to use AOD-9604 for cartilage, muscle, and joint repair (e.g. osteoarthritis treatment), but as of 2013 experts noted a lack of clinical evidence for any significant tissue-repair benefits. Interestingly, researchers observed that intravenous administration of AOD-9604 sometimes produced a transient euphoric effect in volunteers, leading to a patent filing for its potential use as an antidepressant. Around the same time, AOD-9604 became a subject of controversy in sports. It featured in a 2012 Australian Football League supplements scandal, after which the World Anti-Doping Authority (WADA) clarified in 2013 that AOD-9604 is prohibited for athletes under the “S0 – Non-approved substances” category. Regulatory agencies worldwide have not approved AOD-9604 as a prescription medication, though in 2014 the peptide obtained a “Generally Recognized as Safe (GRAS)” designation in the United States for use as an ingredient in foods/supplements, contingent on the publication of its safety data. Indeed, a comprehensive safety profile was published, and since then AOD-9604 has appeared in some cosmetic and nutraceutical products (for example, as a component of an anti-cellulite cream). Nonetheless, its use remains restricted to research contexts, and it is marketed to laboratories as a research peptide for in vitro or animal experiments investigating fat metabolism, obesity, and regenerative medicine.
Molecular Background of AOD-9604
Structure: AOD-9604 is a peptide consisting of 16 amino acids that correspond to the C-terminus of human growth hormone. The core sequence represents HGH amino acids 177–191, with a tyrosine added to the N-terminus (making the first residue Tyr) and a disulfide bond between two cysteine residues in the sequence. This structure mimics the configuration of the fragment as it exists in native HGH. Notably, the native HGH 176–191 fragment (sometimes just called “HGH Frag 176-191”) itself is 15 amino acids long and begins with a phenylalanine at position 176. In AOD-9604, that phenylalanine is replaced by the added tyrosine at the new N-terminal position, which helps stabilize the fragment against degradation lexology.com . The resulting cyclic structure (due to the cysteine–cysteine bridge) is similar in three-dimensional conformation to the same region within full HGH. By isolating this specific region of the hormone, AOD-9604 was engineered to retain the fragment’s intrinsic bioactivity related to fat metabolism while removing the sections of HGH responsible for growth promotion and insulin regulation.
Mechanism of Action: The rationale for focusing on the 177–191 fragment of HGH came from understanding HGH’s role in fat metabolism. Human growth hormone has well-documented effects on adipose tissue: it can stimulate lipolysis (breakdown of fats) and inhibit lipogenesis, leading to reduction of fat cell size and fat mass. These effects are partly mediated by HGH binding to its receptors on adipocytes and triggering downstream enzymes – for example, activating hormone-sensitive lipase and inhibiting acetyl-CoA carboxylase in fat cells, as shown in animal studies. However, HGH also has broad systemic effects, notably raising levels of insulin-like growth factor 1 (IGF-1) from the liver and affecting carbohydrate metabolism (sometimes causing insulin resistance or changes in blood sugar). The 177–191 fragment was hypothesized to separate the lipid metabolism function of HGH from its other endocrine functions. In vitro experiments and animal studies confirmed that this fragment indeed mimics the fat-burning activity of the intact hormone: for example, a fragment (AOD-9401, an earlier variant of AOD-9604) was found to stimulate lipolytic enzymes and release of glycerol from adipocytes similar to full HGH, but without activating pathways that lead to IGF-1 production or hyperglycemia.
At the molecular level, AOD-9604’s mechanism is still being elucidated. It does not function like a typical HGH agonist; in fact, it does not measurably bind or activate the human GH receptor in the same way the full hormone does (hence no increase in IGF-1) jofem.org consensus.app . Instead, AOD-9604 appears to exert its effects directly on adipose tissue metabolism. Research in obese rodent models suggests that AOD-9604 may interact with metabolic regulators in fat cells. One study reported that chronic administration of AOD-9604 increased expression of β3-adrenergic receptors in fat tissue of obese mice. β3-adrenoceptors are known to promote lipolysis in adipocytes, especially in rodents, and their upregulation could enhance fat burning. This aligns with the observation that AOD-9604’s fat-reducing effect is blunted in genetically modified mice lacking β3-adrenergic receptors. Additionally, as noted, AOD-9604 triggers key enzymes: it activates hormone-sensitive lipase (which releases stored fat) and inhibits acetyl-CoA carboxylase (reducing fat synthesis) in fat cells. The net result is increased breakdown of triglycerides and mobilization of fatty acids from adipose tissue. Importantly, these metabolic actions come without the counter-regulatory side effects of HGH. Unlike full HGH, AOD-9604 in studies did not cause hyperglycemia, did not induce insulin resistance, and did not raise IGF-1 – indicating a selective metabolic profile jofem.org.
From a chemical standpoint, AOD-9604 is notable for being orally bioavailable (unusual for peptides). In early trials, it was administered as an oral capsule. Pharmacokinetic studies in animals showed that while AOD-9604 is rapidly degraded in the bloodstream (it has a short half-life on the order of minutes in plasma jofem.org ), a fraction of orally ingested peptide can be absorbed intact. In pigs, for instance, oral dosing led to detectable plasma levels of AOD-9604, though at lower concentrations than equivalent intravenous dosing. This property made AOD-9604 attractive as an oral drug candidate compared to injectable peptide drugs. The fragment’s stability is partly thanks to the modifications (N-terminal Tyr and internal disulfide loop). Still, it undergoes quick enzymatic breakdown into shorter fragments in vivo. Researchers identified several metabolites of AOD-9604 in blood and urine, mostly truncations of the peptide, which were not found to have off-target toxic effects jofem.org.
Research and Current Scientific Findings
Preclinical Studies (In Vitro and Animal Models) From its inception, AOD-9604 was extensively tested in lab models to validate its efficacy and safety. Cell Culture Experiments confirmed that AOD-9604 could influence fat cells. Adipocyte studies demonstrated that treating fat cells with the peptide increased lipolytic activity (glycerol release) comparable to full HGH. Unlike HGH, the fragment did not activate pathways leading to cell proliferation, consistent with its lack of growth effect.
In rodent models of obesity, AOD-9604 showed significant fat-reducing effects. A pivotal study in obese Zucker rats (a genetic model of obesity) found that daily oral administration of AOD-9604 (500 μg/kg) for 19 days reduced the rats’ weight gain by over 50% compared to controls. Treated rats gained only ~16 g of body weight vs ~36 g in untreated obese rats over that period, despite consuming the same diet. Importantly, analysis of adipose tissue confirmed enhanced fat breakdown in the AOD-9604 group, evidencing a direct lipolytic effect in vivo. Remarkably, these metabolic benefits were achieved without the adverse glucose effects associated with HGH. In the same rat study, researchers performed euglycemic clamp tests and found that unlike chronic HGH exposure, chronic AOD-9604 did not impair insulin sensitivity. Blood glucose and insulin dynamics remained normal in AOD-9604–treated rats, supporting the idea that the fragment bypasses HGH’s diabetogenic side effects.
Another study in obese mice echoed these findings. Mice treated with AOD-9604 (via injection) for 14 days had significant reductions in body weight and body fat compared to saline-treated controls pmc.ncbi.nlm.nih.gov . The weight loss from AOD-9604 was similar in magnitude to that caused by full HGH in these mice pmc.ncbi.nlm.nih.gov . Detailed examination showed reductions in fat pad mass and adipocyte size in treated animals. Notably, when these experiments were repeated in genetically engineered mice lacking β3-adrenergic receptors (key mediators of fat metabolism in rodents), the effects of AOD-9604 were diminished – suggesting AOD-9604 may work in part through the sympathetic β3-adrenergic pathway. Even in those knockout mice, however, some residual fat-loss effect was observed, indicating the peptide might have multiple mechanisms of promoting lipid turnover.
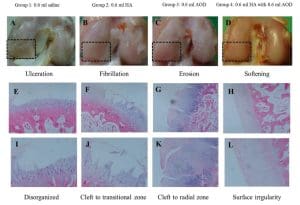
Beyond obesity, preclinical research has investigated other potential uses. Cartilage and Joint Repair is one such area, given some overlapping pathways between metabolism and growth. In a notable 2015 study, AOD-9604 was tested for osteoarthritis therapy in a rabbit model of knee arthritis. Researchers induced osteoarthritis in rabbits and then gave intra-articular injections of AOD-9604, with or without hyaluronic acid (HA), weekly for four weeks. The results were encouraging: rabbits treated with AOD-9604 showed improved cartilage integrity compared to controls. Joint surfaces had less erosion and smoother morphology, and histological scores of cartilage health were significantly better in the AOD-9604 group. The best outcomes were seen in the combination of AOD-9604 + HA, which had more robust cartilage regeneration than either AOD-9604 or HA alone. The lameness (limping) period of the rabbits was also shortest in the combined treatment group. Figure 1 below illustrates some findings from this study – showing the knee joint cartilage from different treatment groups. Group 1 (placebo saline) had severe cartilage ulceration and disorganization, whereas Group 3 (AOD-9604 alone) and Group 4 (AOD-9604 + HA) showed progressively healthier cartilage structure with fewer lesions.
Figure 1: Gross and microscopic cartilage findings in a rabbit osteoarthritis model treated with AOD-9604. Panels A–D (top) show gross morphology of knee joint surfaces for Group 1 (saline control), Group 2 (hyaluronic acid alone), Group 3 (AOD-9604 alone), and Group 4 (AOD-9604 + HA). Groups 1–3 exhibit ulceration, fibrillation, and erosion of cartilage, respectively, while Group 4 shows only mild surface irregularities. Panels E–L (bottom) are histology images (H&E staining) corresponding to each group, indicating that Group 1 had disorganized cartilage with chondrocyte loss, whereas Groups 3 and 4 retained more normal cartilage architecture. Group 4 in particular shows improved cartilage thickness and cellular organization, consistent with regenerative effects. These preclinical results opened the door to considering AOD-9604’s role in tissue repair and regenerative medicine. However, it must be emphasized that no published clinical trials to date have confirmed these benefits in humans. The evidence for joint repair is currently limited to animal models. Similarly, lab studies have explored whether AOD-9604 or related fragments have anti-inflammatory or even anti-cancer properties (for instance, one 2022 study examined using the 176–191 fragment as a carrier to enhance delivery of chemotherapy to breast cancer cells). Such applications remain very experimental.
Human Clinical Trials in Obesity
The most extensive research on AOD-9604 in humans has been in the context of weight loss and obesity. Metabolic Pharmaceuticals ran multiple Phase I and Phase II trials in the early 2000s. The trials investigated oral AOD-9604 in overweight or obese adults, assessing both its safety/tolerability and its efficacy in reducing body weight. One of the first placebo-controlled trials was a 12-week study in roughly 300 obese individuals, testing several different doses of AOD-9604 (ranging from 0.25 mg up to 30 mg daily). The standout result from that study was that a relatively low dose (1 mg per day) produced the greatest weight loss. Subjects taking 1 mg daily lost on average 2.8 kg over 12 weeks, compared to about 0.8 kg in the placebo group. This was a statistically significant difference and amounted to roughly triple the placebo weight loss. Interestingly, higher doses (5, 10, 20, 30 mg) did not outperform the 1 mg dose – indicating a possible bell-shaped or plateau dose-response curve. The rate of weight loss in the 1 mg group was steady throughout the trial (about 0.2–0.3 kg per week) with no sign of early plateau. In addition to weight reduction, there were modest improvements in metabolic markers: treated subjects showed small but consistent improvements in cholesterol profiles and a decrease in the number of individuals with impaired glucose tolerance. Crucially, AOD-9604 did not cause the side effects typical of some obesity drugs. The trial report noted the drug was “very well tolerated with no evidence of the side effects commonly experienced with existing obesity drugs” news-medical.net . In particular, unlike appetite suppressants, AOD-9604 did not cause cardiovascular stimulation (e.g. no increase in heart rate or blood pressure was observed), and unlike lipase inhibitors, it did not cause gastrointestinal distress news-medical.net .
Following this positive Phase II trial, a larger and longer Phase IIb trial was conducted – known as the “OPTIONS Study.” This trial enrolled about 500 obese subjects and treated them for 24 weeks (6 months) to see if the weight loss would be more substantial over a longer period. Unfortunately, the 24-week study did not meet its primary efficacy endpoint. While there was some weight loss observed, the difference between AOD-9604 and placebo was not statistically significant at 24 weeks in the overall study population. An average weight loss of only ~1–2 kg was noted, which was not enough to justify continued development, especially given the placebo group also lost weight with diet and exercise counseling. As a result, Metabolic Pharmaceuticals announced in early 2007 that AOD-9604 would be dropped as an obesity drug candidate lexology.com . The consensus from these trials was that AOD-9604’s weight loss effect is real but modest – potentially useful as an adjunct but not a stand-alone obesity cure. Some observers have compared it to existing obesity medications: for example, the 2.8 kg weight loss at 12 weeks on AOD-9604 (1 mg) was slightly higher than that seen with orlistat (a then-leading obesity drug) in similar timeframe, but AOD-9604 lacked orlistat’s side effects. Despite these encouraging signals, the inability to produce larger sustained weight reduction led to a pause in clinical development.
Safety Profile and Tolerability
One consistently positive finding across all human studies was the excellent safety and tolerability of AOD-9604. In both short-term and long-term trials, AOD-9604 did not cause significant adverse effects at the doses tested. A comprehensive review of six clinical trials (spanning doses from 0.25 mg to 30 mg, with treatment durations up to 6 months) concluded that “AOD9604 has a very good safety and tolerability profile, without any of the adverse effects associated with full-length hGH treatment.” consensus.app . Unlike full HGH, the fragment caused no elevation in IGF-1 and no signs of acromegaly-like effects (such as tissue growth, edema, joint pain). In a dedicated safety study, serum IGF-1 levels remained unchanged in subjects receiving AOD-9604, confirming that the peptide does not act through the HGH/IGF axis jofem.org . Additionally, detailed metabolic panels showed AOD-9604 had no negative impact on carbohydrate metabolism: in fact, oral glucose tolerance tests indicated no impairment of glucose handling during or after AOD-9604 therapy consensus.app . Vital signs, ECGs, and laboratory markers remained normal. Some trial participants did report mild, transient side effects (e.g. headache or nasopharyngitis were mentioned in some trial reports), but rates were similar to those in the placebo group, and no specific pattern of concern emerged. Even liver and kidney function tests showed no difference from placebo over 6 months. This benign safety profile is in line with animal toxicology studies, where high-dose chronic administration in rodents and primates caused no organ toxicity and no evidence of mutagenicity or carcinogenesis (battery of genotoxicity assays were all negative). Given this data, in 2014 an expert panel in the U.S. accepted AOD-9604 as GRAS for use in food supplements. This was a somewhat unusual step for a peptide, reflecting that at the time it was being positioned as a weight-management supplement ingredient rather than a drug. It’s worth noting that regulatory views can evolve – for instance, in some countries, any analog of HGH might be regulated as a drug. Nonetheless, to date there have been no serious safety signals with AOD-9604 in research settings. Contraindications have not been formally established since the peptide isn’t an approved therapy, but researchers advise caution in extrapolating use to humans with certain conditions. For example, even though AOD-9604 does not raise IGF-1, its use in individuals with active cancer is not recommended (HGH itself can promote some cancers, so fragments should be used cautiously until more is known). Likewise, pregnant or lactating individuals, children, or those with severe metabolic disease were excluded from trials, so no data exists for those groups – these would be considered off-limits for any hypothetical use. Overall, AOD-9604’s side effect profile appears minimal in the contexts studied; in fact, the predominant critique of the peptide is not any toxicity, but rather its limited efficacy in obesity when used alone lexology.com . Comparison with HGH and Other HGH Fragments
AOD-9604 is often compared to two related substances: full-length human growth hormone (HGH) and the unmodified HGH fragment 176–191. It’s important for researchers to understand the distinctions: Versus HGH: Full-size human growth hormone (191 amino acids) is a potent metabolic and growth-regulating hormone. Pharmacologically, HGH can induce fat loss, increase muscle mass, and alter metabolism, but it also elevates IGF-1 and has side effects like insulin resistance, edema, joint pain, and potential tumor-promoting effects with long-term misuse. AOD-9604 was explicitly created to separate HGH’s lipolytic effects from these unwanted actions. The clinical and biochemical data confirm that AOD-9604 indeed does not behave like HGH in areas such as IGF-1 induction or anabolic effects. AOD-9604 did not cause any HGH-like adverse effects in trials – for example, no changes in blood glucose or IGF-1 were seen jofem.org . In terms of efficacy, both HGH and AOD-9604 can reduce body fat, but HGH typically causes broader changes (including slight lean mass gain and fluid retention) whereas AOD-9604’s effects seem confined to adipose tissue. An advantage of AOD-9604 is that it can be given orally and remains stable, whereas HGH is a large protein that must be injected and is quickly broken down if swallowed. AOD-9604 is also smaller (molecular weight ~1815 Da) versus HGH (~22,000 Da), which affects tissue penetration and clearance. In summary, AOD-9604 offers a targeted approach to mimic HGH’s fat loss benefit without involving the HGH receptor. This means significantly fewer systemic effects: as one review stated, AOD-9604 “mimics the lipolytic effects of GH without producing growth effects.” pmc.ncbi.nlm.nih.gov . For research purposes, AOD-9604 can be a valuable tool to probe fat metabolism pathways “decoupled” from the broader endocrine effects of HGH. Versus HGH Fragment 176–191 (unmodified): The terms “HGH Frag 176-191” and “AOD-9604” are sometimes used interchangeably in literature, but there is a technical difference. HGH fragment 176–191 refers to the peptide sequence as it exists in the natural hormone (usually a 15-mer peptide). AOD-9604 is a modified version of this fragment (16-mer with modifications) lexology.com . While the unmodified fragment has shown bioactivity in animal studies, it has not undergone the same level of clinical testing. In fact, a Wikipedia review notes that “in contrast to AOD9604, hGH frag 176–191 has not been studied in humans.”. AOD-9604’s developers chose to modify the fragment to improve its drug-like properties – the added tyrosine and disulfide bond likely confer better stability and half-life. If a researcher encounters “HGH Fragment 176-191” in a catalog, it may or may not be identical to AOD-9604 depending on whether it includes the tyrosine and the cysteine bridge. The biological function, however, is intended to be the same – both are aimed at the fragment’s inherent lipolytic activity. Another HGH-derived fragment in a similar vein is HGH frag 176-191 (non-acetylated) or acetylated frag. Some suppliers offer a peptide corresponding to AA 176–191 (16 amino acids if acetylated at the N-terminus). These are experimental and, like AOD-9604, fall under research chemicals. AOD-9604 remains the fragment variant with the most robust data behind it, given the published animal and human studies. So, when comparing AOD-9604 to a generic “HGH Frag,” one should ensure they understand whether they refer to the same modified sequence. For practical purposes, the scientific consensus gleaned from AOD-9604 studies is often extrapolated to HGH 176–191 fragments generally, since the core sequence is the same. Potential Benefits and Scientific Implications
Though AOD-9604 is not an approved therapy, its research journey has yielded insights and potential applications:
- Anti-Obesity Effects: AOD-9604 has demonstrated an ability to reduce body fat and body weight modestly, especially in combination with diet/exercise. It could be potentially useful as a supportive agent for weight management. Its chief benefit is a targeted fat metabolism effect without systemic hormonal disruptions. For instance, abdominal fat reduction was noted to be preferential in some analyses of AOD-9604 data, suggesting it may particularly help with visceral fat (a dangerous fat linked to metabolic syndrome). However, given the lack of robust efficacy in large trials, any benefit is likely moderate. Researchers are still interested in how AOD-9604 might be combined with other interventions (e.g., with diet, exercise, or other medications) to synergistically combat obesity.
- Metabolic Health: Beyond weight loss per se, AOD-9604 might impart metabolic improvements. The trial evidence of improved cholesterol and glucose tolerance, while preliminary, hints that by promoting fat breakdown, AOD-9604 could favorably influence lipid profiles and insulin sensitivity. In obese animal models, chronic AOD-9604 prevented the decline in insulin sensitivity that is usually seen with HGH. This raises the prospect that AOD-9604 or similar fragments could be explored for type 2 diabetes or dyslipidemia adjunct therapy, where weight loss and fat redistribution help improve metabolic parameters.
- Joint and Tissue Repair: As discussed, preclinical evidence suggests AOD-9604 may stimulate aspects of tissue repair, such as cartilage regeneration. The mechanism isn’t clear – it might involve localized anti-inflammatory effects or influencing mesenchymal stem cells. Some companies have shown interest in formulating AOD-9604 for osteoarthritis (e.g., combining it with hyaluronic acid for joint injections). If these findings translate to humans, it could represent a novel therapeutic avenue for degenerative joint disease. Caution is warranted until clinical trials confirm efficacy, but this remains an intriguing benefit under investigation. It is a good example of how a peptide initially developed for one purpose (fat loss) might have pleiotropic effects in other tissues.
- Safety Advantages: One of the “benefits” frequently highlighted by researchers is safety – which is rare to emphasize, but in the context of anti-obesity agents (many of which have serious side effects), AOD-9604’s clean safety record is a major plus. It does not cause the side effects seen with many weight loss drugs like cardiovascular strain or psychiatric issues news-medical.net . It also avoids the long-term risks of HGH therapy (like diabetes or carpal tunnel syndrome) because it doesn’t trigger the same pathways. This favorable safety margin means that if AOD-9604’s efficacy could be slightly enhanced (perhaps via analogs or combination therapy), it could become a valuable tool – essentially, a therapy that nudges the body to burn fat naturally, without the pitfalls of hormonal therapy.
- Scientific Tool: For researchers, AOD-9604 serves as a probe to study fat metabolism. It allows the dissection of HGH’s actions: using AOD-9604 in experimental settings can help differentiate which effects on adipose tissue are direct (fragment-mediated) versus which require the full hormone and IGF-1. In metabolic research, AOD-9604 has been used to investigate pathways of lipolysis, adipocyte differentiation, and beta-adrenergic signaling. Its availability as a research peptide has spurred studies in endocrinology and pharmacology to design improved peptides that target obesity.
Known Limitations and Contraindications
All findings indicate that AOD-9604 is well-tolerated, but researchers should remain aware of its limitations:
- Limited Efficacy: The weight loss achievable with AOD-9604 alone is moderate. It is not a potent anorectic or a metabolic panacea. Thus, any research or potential future use in humans would likely see it as part of a comprehensive regimen, not a stand-alone “fat melting” cure. This also means that in clinical research, detecting significant effects may require large sample sizes or specific sub-populations (e.g., those with certain obesity phenotypes). Indeed, one post-hoc observation was that AOD-9604’s effect might be more pronounced in people with particular patterns of fat distribution (like high visceral fat), though this needs confirmation.
- Regulatory Status: As a non-approved substance, AOD-9604 cannot be used in or on humans outside research protocols. Any mention of its use must clarify that it’s experimental. For athletes or doping-controlled populations, AOD-9604 is banned (even though it’s not a traditional anabolic agent, its unapproved status places it on WADA’s prohibited list).
- Contraindications (Theoretical): While no formal contraindications exist (since it’s not prescribed), researchers typically avoid using growth-factor-related peptides in certain scenarios. For example, in cancer research, one must be cautious: AOD-9604 itself has not shown pro-tumor activity (if anything, fragments might inhibit some tumor pathways, as suggested by exploratory cancer studies), but given HGH can stimulate cell proliferation, fragments should be tested thoroughly for safety in oncology contexts. Similarly, individuals with severe organ failure or endocrine disorders were never exposed to AOD-9604 in trials, so those remain unknown territories. Until more data emerges, one should assume that AOD-9604 could have unknown interactions in unstudied disease states.
- Lack of Long-Term Data: Human trials up to 6 months showed no harm, but very long-term effects (years of use) have not been studied. With any metabolic peptide, there’s a question of whether the body develops tolerance or adaptive responses. Some speculate that the diminishing returns at higher doses might indicate a feedback mechanism (perhaps high doses desensitize adipose response). There’s also no data on using AOD-9604 in children or adolescents (who might benefit from fat-loss in severe obesity, but their growth considerations would be important).In conclusion, AOD-9604 has carved out a niche in scientific research as a selective lipolytic peptide with a strong safety profile. It offers a glimpse of how we might harness the beneficial portion of a hormone’s activity while minimizing risks. The history of AOD-9604 – from an obesity drug hopeful to a shelved project, and now to a research and supplement curiosity – underscores the challenges in translating fat-burning biology into effective therapy. Nonetheless, ongoing studies continue to shed light on AOD-9604’s potential: whether as a standalone research tool or possibly combined with other agents (like in the cartilage repair scenario). For licensed researchers delving into metabolism or regenerative medicine, AOD-9604 remains an intriguing compound worthy of further investigation, always keeping in mind that any in vivo use is experimental and subject to ethical and regulatory oversight.
References
Ng, F. M., Sun, J., Sharma, L., Libinaka, R., Jiang, W. J., & Gianello, R. (2000). Metabolic studies of a synthetic lipolytic domain (AOD9604) of human growth hormone. Hormone Research, 53(6), 274–278. Heffernan, M., et al. (2000). Molecular and cellular actions of a structural domain of human growth hormone (AOD9401) on lipid metabolism in Zucker fatty rats. Journal of Molecular Endocrinology, 25(3), 287–298.
Misra, M., & Doshi, P. (2013). Obesity pharmacotherapy: current perspectives and future directions. Curr Opin Endocrinol Diabetes Obes, 20(5), 384–401.
Valentino, M. A., Lin, J. E., & Waldman, S. A. (2010). Central and peripheral molecular targets for anti-obesity pharmacotherapy. Clinical Pharmacology & Therapeutics, 87(6), 652–662
Stier, H., Vos, E., & Kenley, D. (2013). Safety and tolerability of the hexadecapeptide AOD9604 in humans. J Endocrinol Metab, 3(1-2), 7–15
Moré, M. I., & Kenley, D. (2014). Safety and metabolism of AOD9604, a novel nutraceutical ingredient for improved metabolic health. J Endocrinol Metab, 4(3), 64–77.
Kwon, D. R., & Park, G. Y. (2015). Effect of intra-articular injection of AOD9604 with or without hyaluronic acid in a rabbit osteoarthritis model. Annals of Clinical & Laboratory Science, 45(4), 426–432. Metabolic Pharmaceuticals Ltd. (2004). Obesity drug codenamed AOD9604 highly successful in trials.
Phillips Ormonde Fitzpatrick. (2013). AOD-9604: patents, peptides, performance and…cellulite? (Lexology). Wikipedia. (2025). HGH Fragment 176–191. Wikipedia, The Free Encyclopedia. Citations Favicon AOD-9604: patents, peptides, performance and….cellulite? –
https://www.lexology.com/library/detail.aspx?g=bdb7463f-df5c-419f-9238-6613c56c5c86
https://www.lexology.com/library/detail.aspx?g=bdb7463f-df5c-419f-9238-6613c56c5c86
Safety and Tolerability of the Hexadecapeptide AOD9604 in Humanshttps://www.jofem.org/index.php/jofem/article/view/157
Does AOD9604 peptide safely promote fat loss in humans …https://consensus.app/results/?q=Does%20AOD9604%20peptide%20safely%20promote%20fat%20loss%20in%20humans%3F&pro=on
https://jofem.org/index.php/jofem/article/viewFile/213/279 Central and Peripheral Molecular Targets for Anti-Obesity Pharmacotherapy – PMC
https://pmc.ncbi.nlm.nih.gov/articles/PMC3136748/
Obesity drug codenamed AOD9604 highly successful in trials https://www.news-medical.net/news/2004/12/16/6878.aspx
Clinical safety and tolerability of AOD9604 in obesity treatment …https://consensus.app/results/?q=Clinical%20safety%20and%20tolerability%20of%20AOD9604%20in%20obesity%20treatment&pro=on
Does AOD9604 peptide safely promote fat loss in humans …https://consensus.app/results/?q=Does%20AOD9604%20peptide%20safely%20promote%20fat%20loss%20in%20humans%3F&pro=on