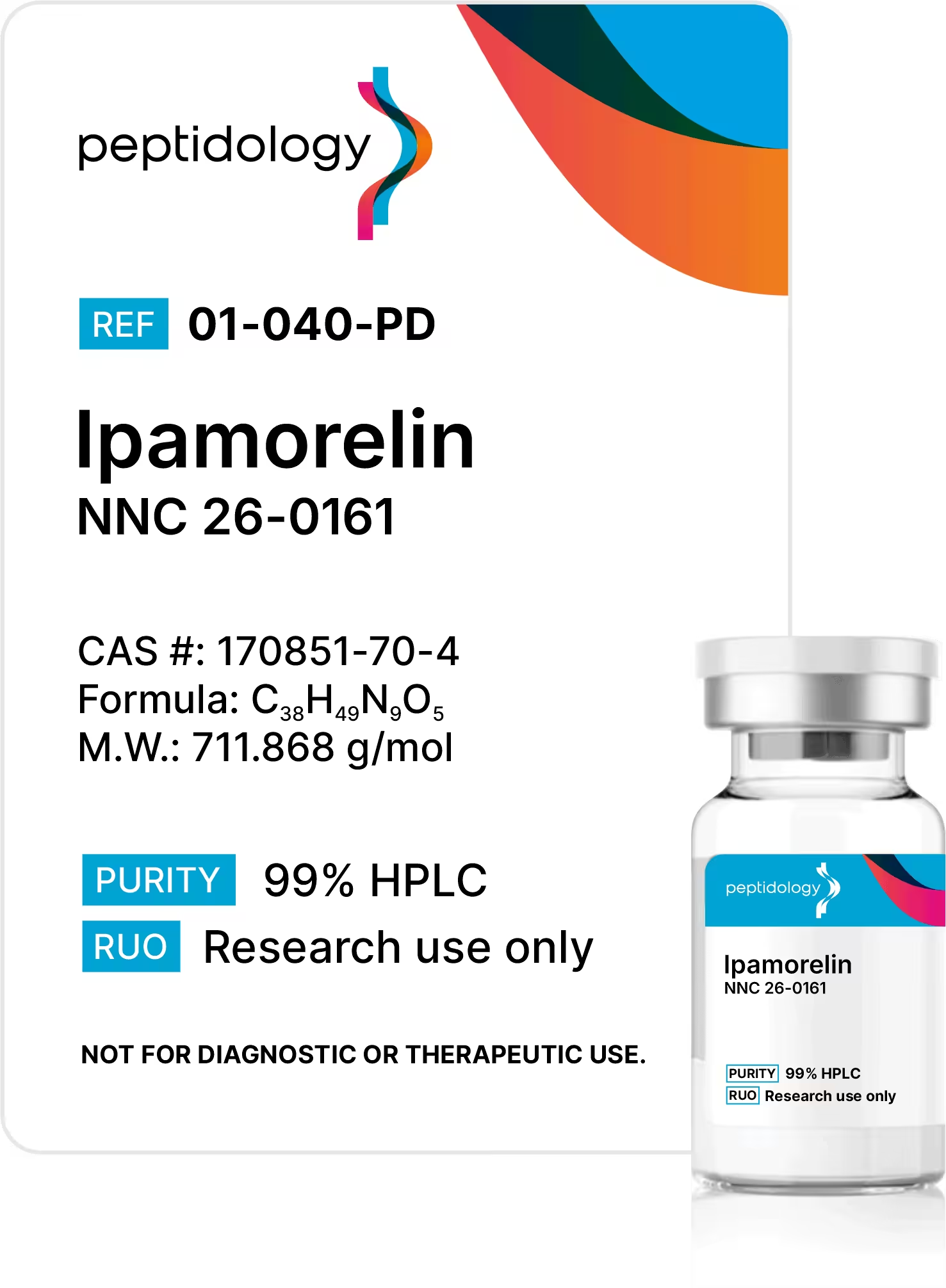
7X Tested
for Advanced Research
Empowering researchers with high-purity, double lab sterility-tested, and endotoxin/elemental-impurity-screened analytical standards built for reproducibility.
Shop now



Purity Is the Baseline — Release Standards Are the Difference
Purity and content testing are important, but they’re only part of a defensible analytical release program.
At Peptidology, each lot is supported by structured, third-party verification—using validated methods and ISO/IEC 17025:2017-accredited laboratory testing—so researchers can rely on consistent, well-documented materials for controlled in-vitro work.
What we verify beyond purity/content (lot-specific results provided):
-
Bacterial endotoxin testing (reported per lot) to help reduce background interference in sensitive assays
-
Elemental impurity screening (reported per lot) for trace metals that can confound analytical and bioassay outcomes
-
Microbial contamination testing performed by two independent labs (reported per lot) to support sterility-sensitive workflows
-
A structured multi-point (“7-point”) analytical verification program to support identity, purity, and lot consistency
Why these standards matter for research
Endotoxins and trace contaminants are often not visible and may still affect assay performance, especially in cell-based or high-sensitivity analytical workflows. Likewise, microbial contamination can compromise sterility-dependent processes and create variability across experiments. Our approach focuses on measuring and reporting these factors—not relying on assumptions.
Documentation you can audit
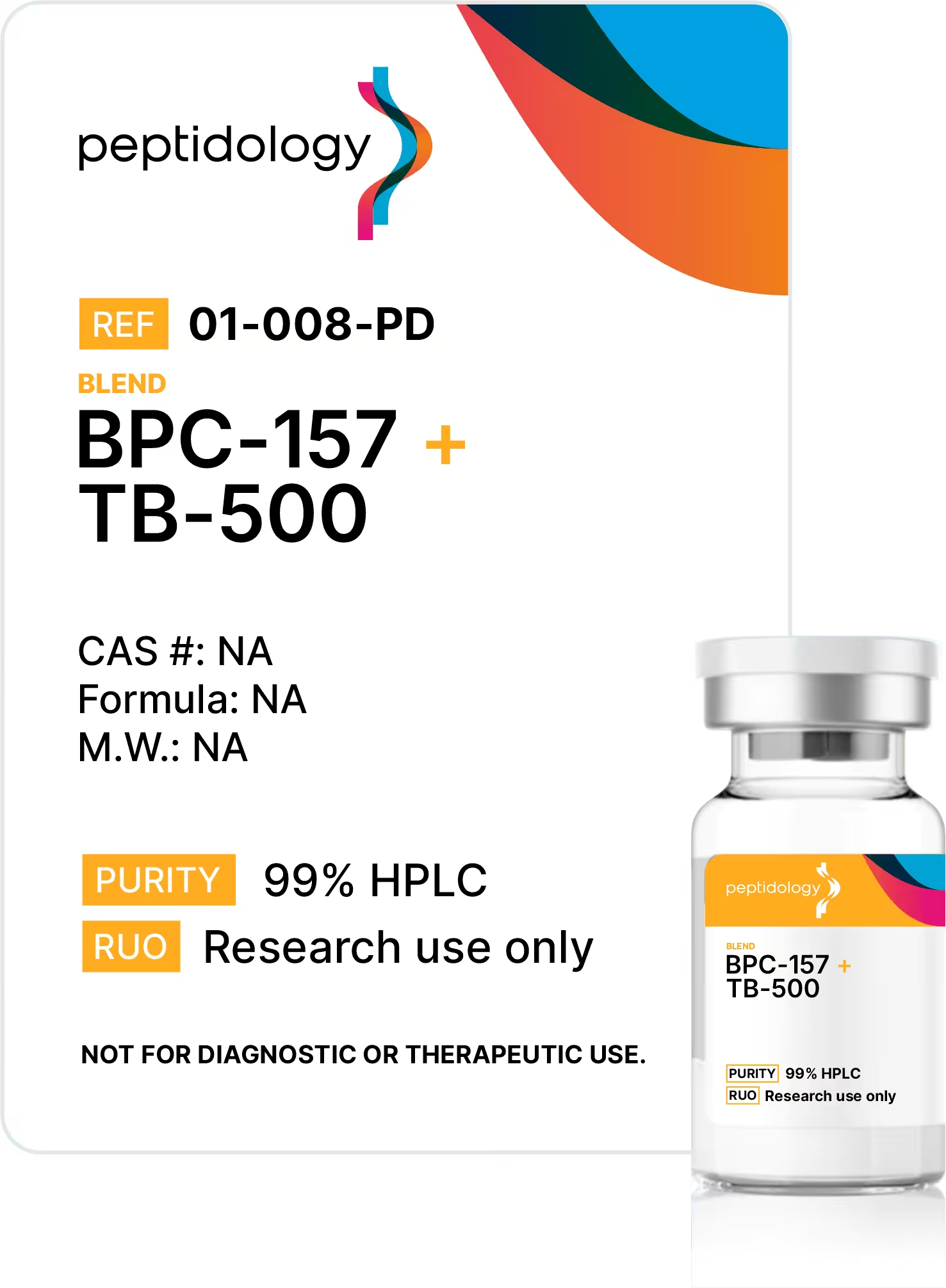
Every lot is backed by a transparent, lot-specific Certificate of Analysis (CoA) with reported results and traceability for key analytical attributes (e.g., purity, content, endotoxin, elemental impurities, and double lab verified microbial testing). No vague claims—just documentation you can compare.
For in-vitro research use only (RUO). Not for human or veterinary use.