6X Tested
for Safer Peptides
Empowering researchers with pure, sterile & endotoxin free peptides engineered for reproducibility.
Shop now

Shop by Category


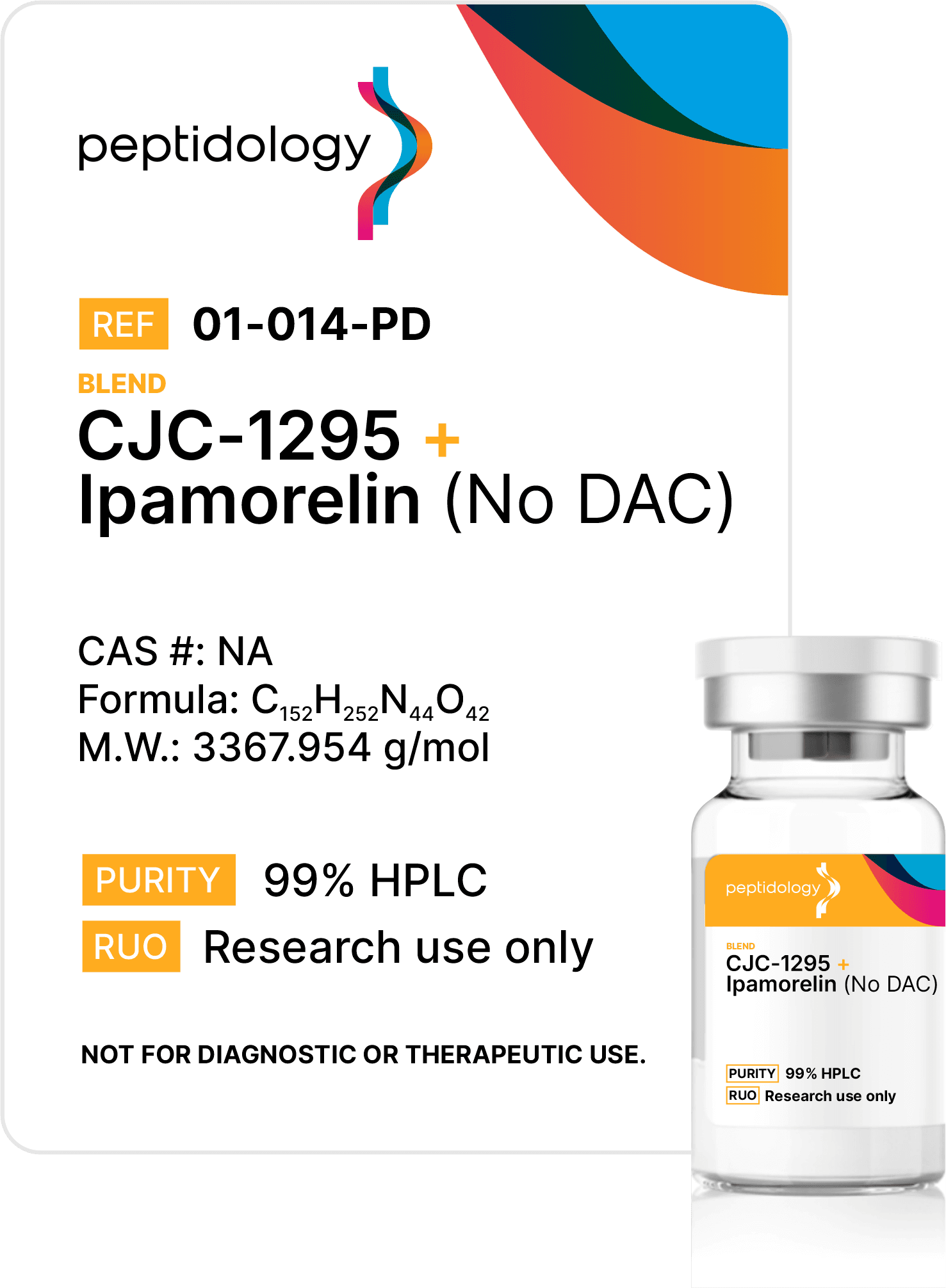
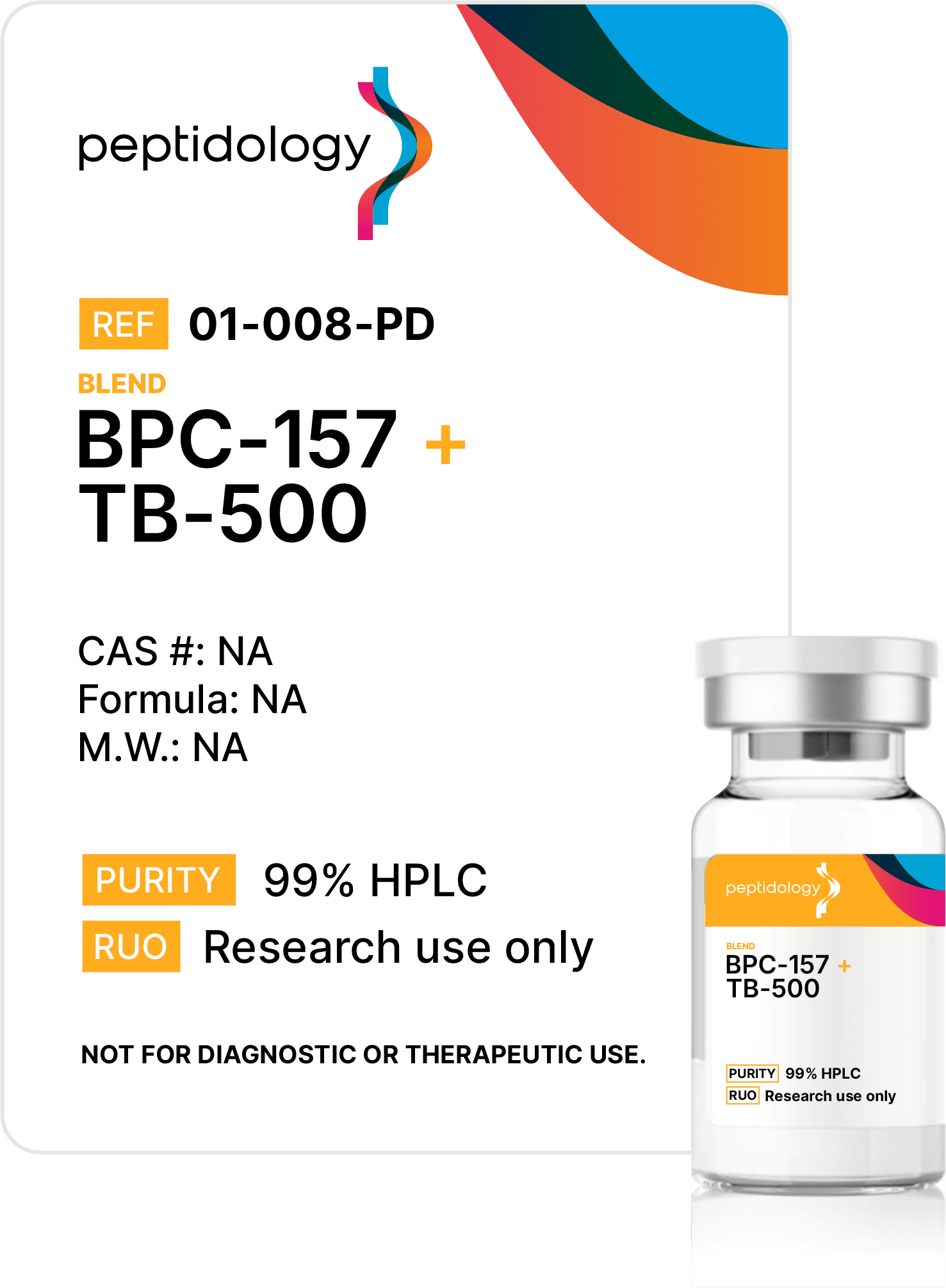
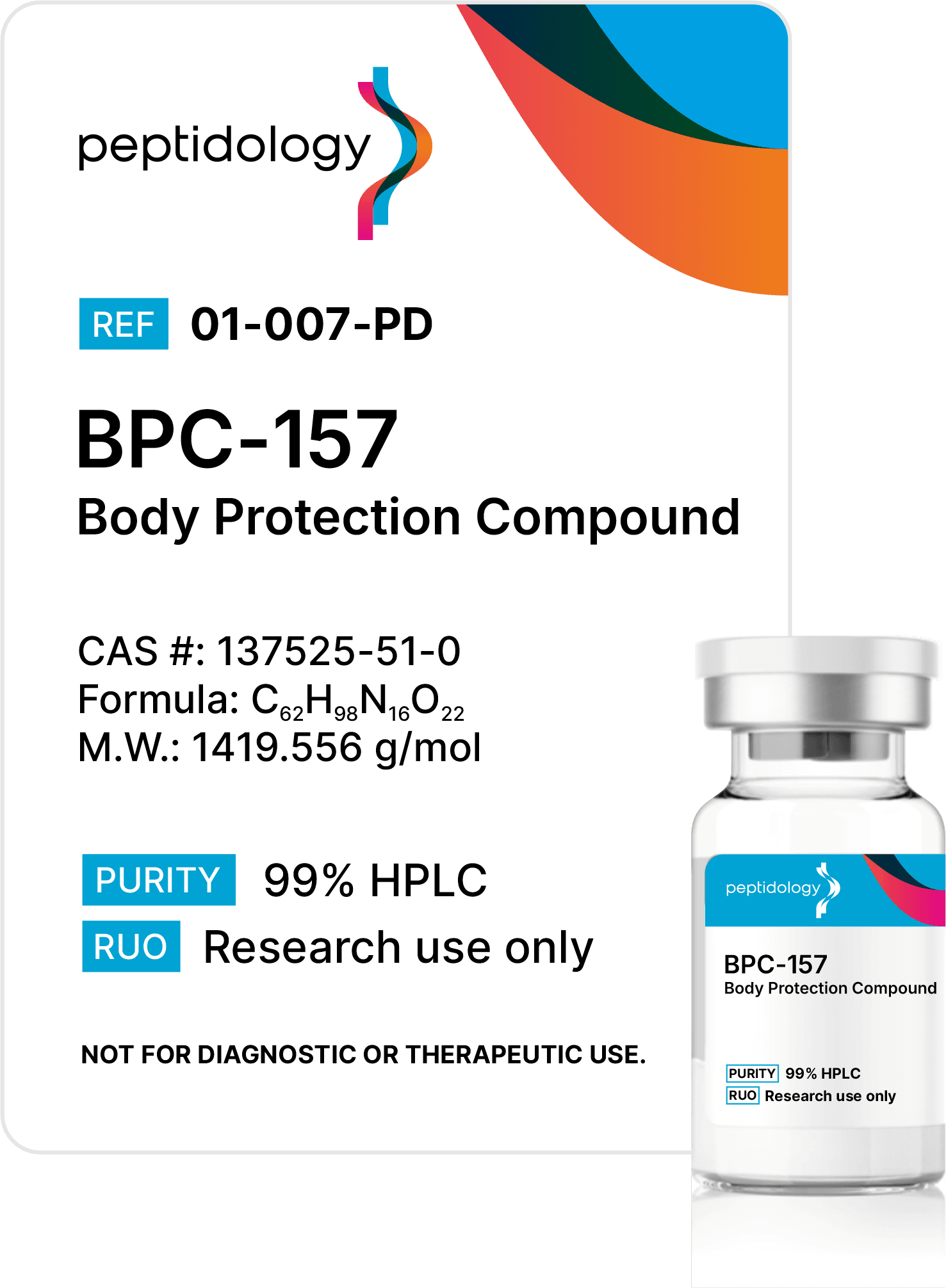
Purity Matters, but it’s not enough
At Peptidology, we go further than purity & content testing, by specifying costly and rigorous additional third-party analysis that includes endotoxin testing to ensure Endotoxin-safe product, heavy metals and sterility verification from an ISO/EIC 17025:2017 accredited lab – critical safeguards most competitors skip.
Endotoxins are invisible bacterial contaminants that can trigger unintended immune responses, even when no visible impurities exist. Our advanced testing protocol eliminates these risk of these threats using medical-grade standards.
Sterility verification ensures every batch is free of viable microbes, preventing contamination that could multiply and ruin experiments. Unlike basic or no filtration at all, our process guarantees sterility.
We back every batch with transparent certificates proving purity, content, endotoxin, and sterility. This isn’t optional – it’s how we build trust. Choose peptides tested to standards others ignore.